Introduction to Clinical Trials
Clinical trials are the backbone of medical science, carefully structured research studies that evaluate new treatments, drugs, or procedures in human participants. These investigations are not conducted in isolation; instead, they follow strict scientific protocols to assess the benefits and risks associated with innovative therapies objectively. In clinical trials, both patients and healthy volunteers contribute vital data that ultimately shape healthcare practices worldwide. By enrolling in clinical trials, participants provide researchers with vital real-world feedback, helping identify side effects and determine whether a new intervention is more effective than existing treatments or even a placebo.
The process of conducting clinical trials unfolds through a precise methodology to ensure both objectivity and reliability. Stringent study designs, such as randomization and blinding, help eliminate bias and strengthen the validity of outcomes. The resulting data serve as the primary evidence guiding regulatory decision-making, allowing agencies and health authorities to approve only those therapies that demonstrate safety and effectiveness. This focus on evidence-based research not only advances scientific understanding but also transforms the standard of care for myriad illnesses and disorders, giving hope to patients facing previously untreatable conditions.
Historical Milestones in Clinical Trials
The evolution of clinical trials is deeply interwoven with some of history’s most significant breakthroughs in medicine. The first recorded clinical trial, conducted in the 18th century by James Lind, explored dietary treatments for scurvy among sailors. This pioneering work not only led to the adoption of citrus fruits in naval diets but also established the methodological principles still in use today. Throughout the 20th century, clinical trials facilitated the development of critical interventions such as the polio vaccine, which eradicated a once-dreaded childhood disease, and the widespread adoption of antibiotics, which revolutionized the treatment of bacterial infections. More recent achievements include the approval of innovative cancer immunotherapies and rapid COVID-19 vaccines, made possible by adaptive and large-scale trial designs.
Each historic milestone underscores the necessity of robust, scientific evaluation before new interventions reach the public. Without the rigor of structured trials, society would remain vulnerable to ineffective or dangerous treatments. The history of clinical trials stands as a testament to the transformative power of well-designed studies to improve public health and change the course of epidemics, saving millions of lives across generations.
Design and Phases of Clinical Trials
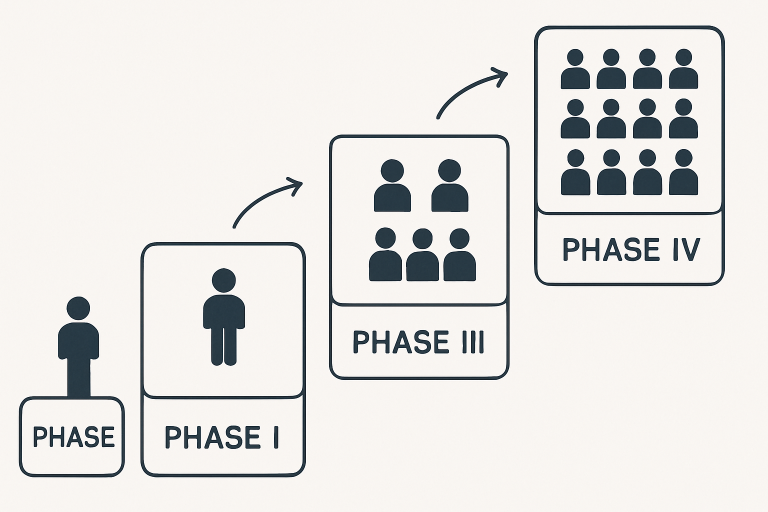
Rather than a single event, the clinical trial process unfolds through well-defined phases, each with distinct objectives, methodologies, and levels of scrutiny. Understanding these phases helps explain how new medical innovations are systematically tested for both safety and effectiveness:
- Phase I: Initial trials involve a small group of healthy volunteers or patients. The primary focus is on establishing a safe dosage range, identifying common side effects, and understanding the drug’s pharmacokinetics in humans.
- Phase II: These studies expand the participant pool and introduce greater diversity, focusing on how well the treatment works for a particular condition while continuing to monitor safety.
- Phase III: This phase involves thousands of participants across multiple research centers and is designed to deliver the most comprehensive evidence on how well a new intervention works compared to standard treatments or placebos. As outlined by the FDA in its guidance on clinical research, this stage plays a pivotal role in determining whether a therapy is ready for broader use. During this period, monitoring for rare or severe side effects becomes even more rigorous, ensuring that any potential risks are identified before developers move forward with regulatory approval.
- Phase IV: Post-approval studies occur after a treatment enters the market, tracking its long-term effects on larger patient populations and identifying rare adverse events that might not have appeared in earlier phases.
This methodical approach ensures that potentially life-saving interventions are introduced responsibly for public use. Meticulous record-keeping, stringent safety monitoring, and ongoing risk-versus-benefit evaluation are essential to trustworthy clinical research.
 Technological Innovations Enhancing Clinical Trials
Technological Innovations Enhancing Clinical Trials
In recent years, technology has revolutionized every step of the clinical trial process, bringing greater speed, precision, and reach to medical research. Artificial intelligence (AI) and large-scale data analytics are now leveraged to efficiently match eligible candidates to trials, predict trial outcomes, and uncover meaningful trends and insights from massive datasets. AI can even help design adaptive trials that adjust in real time based on interim findings, improving both participant safety and scientific value. Predictive modeling minimizes trial failures by refining patient-selection criteria and helping researchers anticipate which groups are most likely to benefit from new therapies.
Beyond AI, the proliferation of wearable health devices, smartphone apps, and remote monitoring tools means clinical data can now be collected in real time, outside clinical settings, and sometimes even passively, increasing both accuracy and participant engagement. Not only does this reduce the burden on participants—who may no longer need to travel for every checkup—but it also enables continuous monitoring of vital signs and early detection of side effects, ultimately enhancing the integrity and depth of the collected data.
Decentralized Clinical Trials: A New Era
Traditionally, most clinical trials were conducted at hospitals or research centers, with participants required to make frequent in-person visits. However, the rise of decentralized clinical trials (DCTs) is fundamentally shifting this paradigm. Enabled by digital health tools, telemedicine, and direct-to-patient drug shipments, DCTs empower participants to complete many assessments from the convenience of their own homes. This shift not only reduces logistical challenges such as travel and time commitments but also allows more people—especially those living in rural locations, individuals with limited mobility, or participants with demanding schedules—to take part in research. By increasing access and flexibility, DCTs support greater diversity and inclusivity, ensuring that underrepresented groups have a seat at the table. Tapping into broader, more representative patient populations improves the real-world relevance of trial results. Importantly, DCTs also enable the collection of more diverse and more frequent data, thereby enhancing the quality of the evidence generated.
Ethical Considerations and Regulatory Frameworks
Ethics are central to clinical research, shaping every stage from study design to final data reporting. As outlined in resources such as the National Institutes of Health, the foundational values of respect for persons, beneficence, and justice ensure that participants are protected at every step of the process. Informed consent remains a core requirement, with individuals voluntarily agreeing to participate only after gaining a clear understanding of potential risks and benefits.
Safeguards such as confidentiality of personal health information, open communication between researchers and participants, and the assurance that participants may withdraw at any time further strengthen these protections. These measures not only uphold ethical standards but also reinforce the trust needed for meaningful research participation. As clinical studies grow more global and complex, strict adherence to ethical guidelines becomes even more important. These principles help maintain the integrity of research, support transparent scientific practices, and ensure that advancements in medicine are achieved responsibly and respectfully.
Conclusion
Clinical trials remain the cornerstone for advancing medical knowledge and improving care worldwide. Driven by ongoing innovation, ethical oversight, and a renewed focus on diversity and accessibility, clinical research continues to provide the scientific evidence needed for physicians, regulators, and patients to make informed decisions. As these efforts expand and evolve, the future promises even greater advances in medicine—benefiting current patients and future generations.